|
Research Article
Objective audiometric evaluation of individuals with type 2 diabetes mellitus: A cross-sectional survey in South West Nigeria
1 Department of Surgery, Ben Carson College of Health and Medical Sciences, Babcock University, Ilishan Remo, Ogun State, Nigeria
2 Department of Diabetes and Endocrinology, Royal Oldam Hospital, Northern Alliance NHS Foundation Trust, Oldam, Greater Manchester, England
3 Department of Family Medicine, Ben Carson College of Health and Medical Sciences, Babcock University, Ilishan Remo, Ogun State, Nigeria
4 Department of Surgery, Faculty of Medicine, Idi Araba University of Lagos, Nigeria
5 Olabisi Onabanjo University Teaching Hospital, Sagamu, Ogun State, Nigeria
6 Junior Clinical Fellow in General Medicine, University Hospitals Coventry and Warwickshire NHS Trust, West Midlands, England
Address correspondence to:
Moses Ayodele Akinola
Department of Surgery, Ben Carson College of Health and Medical Sciences, Babcock University, Ilishan Remo, Ogun State,
Nigeria
Message to Corresponding Author
Article ID: 100012O04MA2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Akinola MA, Idowu A, Ladele AE, Bamigboye BA, Olusoga-Peters O, Adesegun O, Somefun OA. Objective audiometric evaluation of individuals with type 2 diabetes mellitus: A cross-sectional survey in South West Nigeria. Edorium J Otolaryngol 2024;6(2):1–9.ABSTRACT
Aims: Diabetes mellitus affects more than 450 million people around the world. The relationship between diabetes and sensorineural hearing loss has been an area of interest. The aim of this study is to evaluate hearing loss, among type 2 diabetes diagnosed in the endocrinology unit of a private tertiary health facility in South West Nigeria.
Methods: This was a hospital-based cross-sectional study carried out among type 2 diabetic patients and normal healthy non-diabetic individuals as control. A total of 120 participants were recruited for the test and control. Otoacoustic emission and auditory steady-state response audiometry were carried out on all the participants.
Result: The total number of study participants was 120 (60 for test and control) with an age range of 30–79 years for the test and 20–80 years for the control. Otoacoustic emission showed passes for both ears, 61.7% pass in both ears for the test group and 76.7% pass in both ears for the control group. 21.7% of the test and control were referred for both ears. 16.6% of the test group was referred in one ear compared to 1.7% in the control group. Auditory steady-state response (ASSR) audiometry showed mild to profound hearing loss in all test groups while in the control group 20% had normal hearing. Moderate to moderately severe hearing loss in at least one ear was 38.4% in the test ear compared to 16.7% in the control group.
Conclusion: Hearing loss is an established complication of type 2 diabetes. Therefore, it is necessary to incorporate regular hearing screening for patients with diabetes for early detection, counseling, and rehabilitation for those with disabling hearing loss.
Keywords: Otoacoustic emission, Stable state auditory response audiometry, Type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus is considered by many as the “disease of the twentieth century,” affecting more than 450 million people worldwide, with far-reaching consequences that span age, sex, creed, socioeconomic class, and geographical location [1],[2]. It is a global pandemic that requires definitive action to control and prevent long-standing complications. Type 2 diabetes accounts for more than 90% of the disease burden [3].
More commonly than not, long-standing poorly controlled diabetes mellitus results in complications that affect major organs, including the brain, eyes, heart, kidneys, and nerves. One complication of diabetes mellitus that is seldom mentioned is sensorineural hearing loss. Diabetes and its associated complications have a debilitating effect on the health and quality of life of affected individuals [4],[5],[6],[7]; however, adding the loss of sense of hearing makes the situation much worse. The relationship between diabetes and sensorineural hearing loss has been an area of interest since it was reported in 1857 by Jordao [8]. Sensorineural hearing loss is caused by damage to sensory cells or nerve fibers (or both) of the inner ear. Sensorineural hearing loss in diabetics is also characterized by gradual, progressive, and bilateral onset, affecting the highest frequencies [9],[10],[11]. Some authors have reported hearing loss of a more sudden onset and can be unilateral [12]. The higher prevalence of sensorineural hearing loss in diabetics is well documented over the past decades [13],[14],[15],[16] and in Nigeria few studies have reported on this disease [17],[18], although a direct causal relationship has not yet been established and some researchers have even found no significant difference in hearing loss between diabetics and non-diabetic controls [19],[20],[21]. It is also known that the duration of the disease and the level of control can affect the development of sensorineural hearing loss.
Long-term diabetics tend to have a more profound degree of hearing impairment than newly diagnosed individuals [22],[23].
Several theories for the mechanisms of sensorineural hearing loss in diabetics have been proposed: diabetic microangiopathy, diabetic neuropathy, and a mixture of both. Chronic hyperglycemia tends to damage the cochlea, which is highly microvascular and sensitive to injury.
There is endothelial proliferation, accumulation of glycoproteins in the intima, and thickness of the basal membrane of the stria vascularis, the main arterial supply to the cochlea [24],[25]. Microangiopathy markers such as elevated serum creatinine levels, microalbuminuria, and higher levels of stromal cell-derived factor 1a (SDF-1a) have been found in diabetics with higher hearing threshold levels [26],[27],[28]. On the other hand, some researchers have found atrophy of spiral ganglion neurons and demyelination of the vestibulocochlear nerve [25], rather than a vasculopathy as the vascular changes are argued to be nonspecific [29].
Auditory brainstem response is an objective measurement of auditory pathway function from the auditory nerve to the mesencephalon and tests synchronous neural function and can estimate hearing sensitivity thresholds. Pure-tone audiometry is a behavioral test used to measure hearing sensitivity. Puretone thresholds (PTTs) indicate the softest sound audible to an individual at least 50% of the time.
Pure tone audiometry is most commonly used in the assessment of hearing acuity [10],[11],[30]. However, other more specific tests such as otoacoustic emission (OAE) and auditory brainstem response audiometry have been used [10],[31].
This is a hospital-based study that used specific tests to evaluate hearing loss among type 2 diabetics in Nigeria. The results will be useful for making comparisons and spurring action toward recognition of hearing loss as a complication of diabetes and to promote early referral to the otolaryngologist.
The objective of this study is to assess hearing loss among type 2 diabetics diagnosed in the endocrinology unit of a private tertiary health facility in southwest Nigeria.
MATERIALS AND METHODS
The study was carried out in the Otolaryngology and Endocrinology units of Babcock University Teaching Hospital, a 140-bed tertiary health institution located in the western part of Nigeria.
Inclusion criteria: All type 2 diabetic patients attending the endocrinology clinic and normal healthy individuals who gave their informed consent.
Exclusion criteria: History of previous ear infection, meningitis, exposure to ototoxic drugs, prolonged exposure to noise, previous ear surgery, and family history of deafness.
Adult diabetics who met the inclusion criteria had their age and sex matched with healthy controls.
The minimum sample size was determined to be 120.
One hundred and twenty participants were recruited and assigned to two groups, each consisting of 60 members. The control group (Group A) consisted of normal and healthy individuals matched for age and sex, and the diabetic group (Group B) consisted of people diagnosed with type 2 diabetes mellitus.
An interviewer-administered questionnaire was designed to obtain sociodemographic information from each participant, along with their hearing history, duration and treatment of diabetes, presence of complications, and history of smoking. A detailed ear examination was performed on all participants. Samples were obtained for the measurement of baseline fasting blood glucose, glycated hemoglobin (HBA1c).
Otoacoustic emission using a handheld interacoustics OAE device and auditory steady-state response audiometry (ASSR) using the interacoustic ABR/ASSR device were carried out in all participants.
Otoacoustic emission tests the integrity of the outer hair cells of the cochlear while ASSR tests the retrocochlear hearing loss and also gives the hearing threshold of the patient. These tests are objective (not dependent on patients response).
World Health Organization hearing loss grading was used to determine the degree of hearing loss in the study participants.
The approval for the study was obtained from the Babcock University Health Research Ethics Committee. Written informed consent was obtained from each participant and their personal details were left anonymous. Data obtained from this study were entered into the Statistical Package for Social Sciences (SPSS) version 21 program and analyzed.
RESULTS
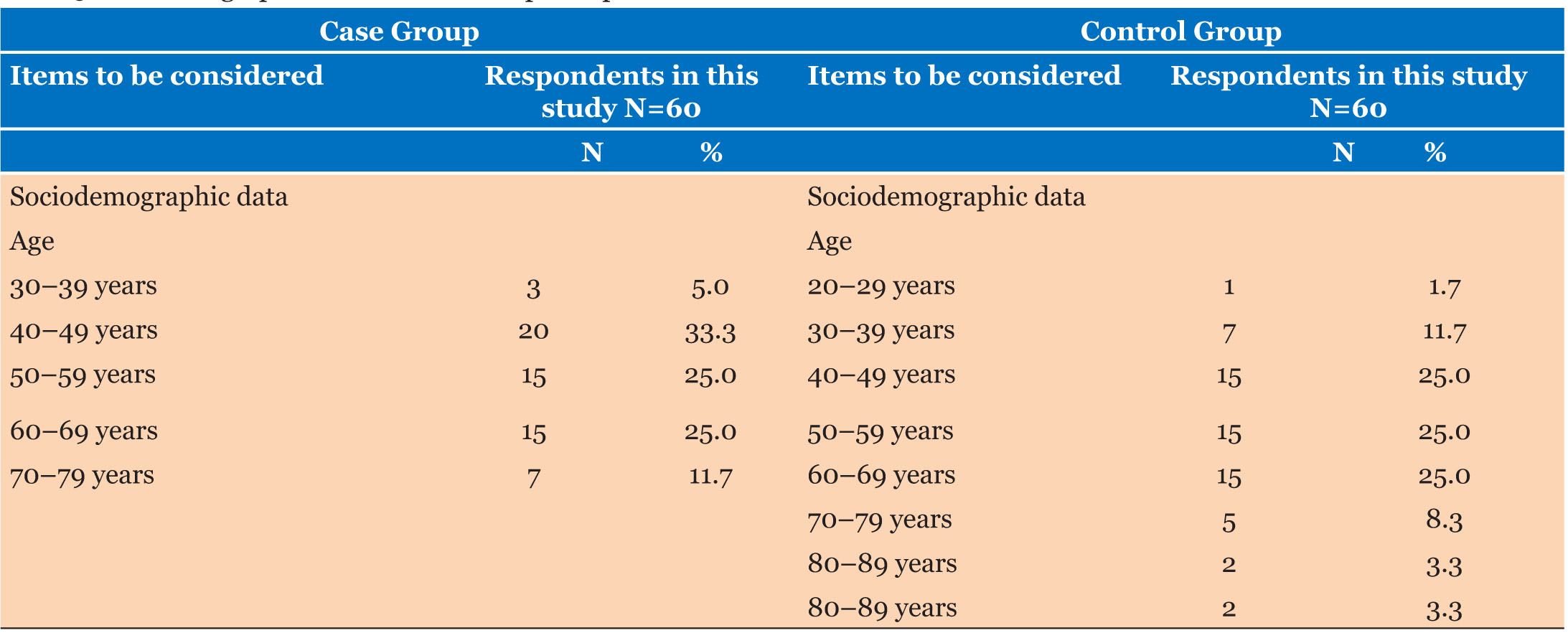
Sociodemographic characteristics
The sociodemographic result revealed that the mean age and standard deviation of the case and control groups was 6.05+1.13 and 5.98±1.33, respectively. The result also revealed that a total of 30 (50.0%) males and 30 (50.0%) females participated in the case group, while 28 (46.7%) males and 32 (53.3%) female participated in the control group.
Plasma glucose level
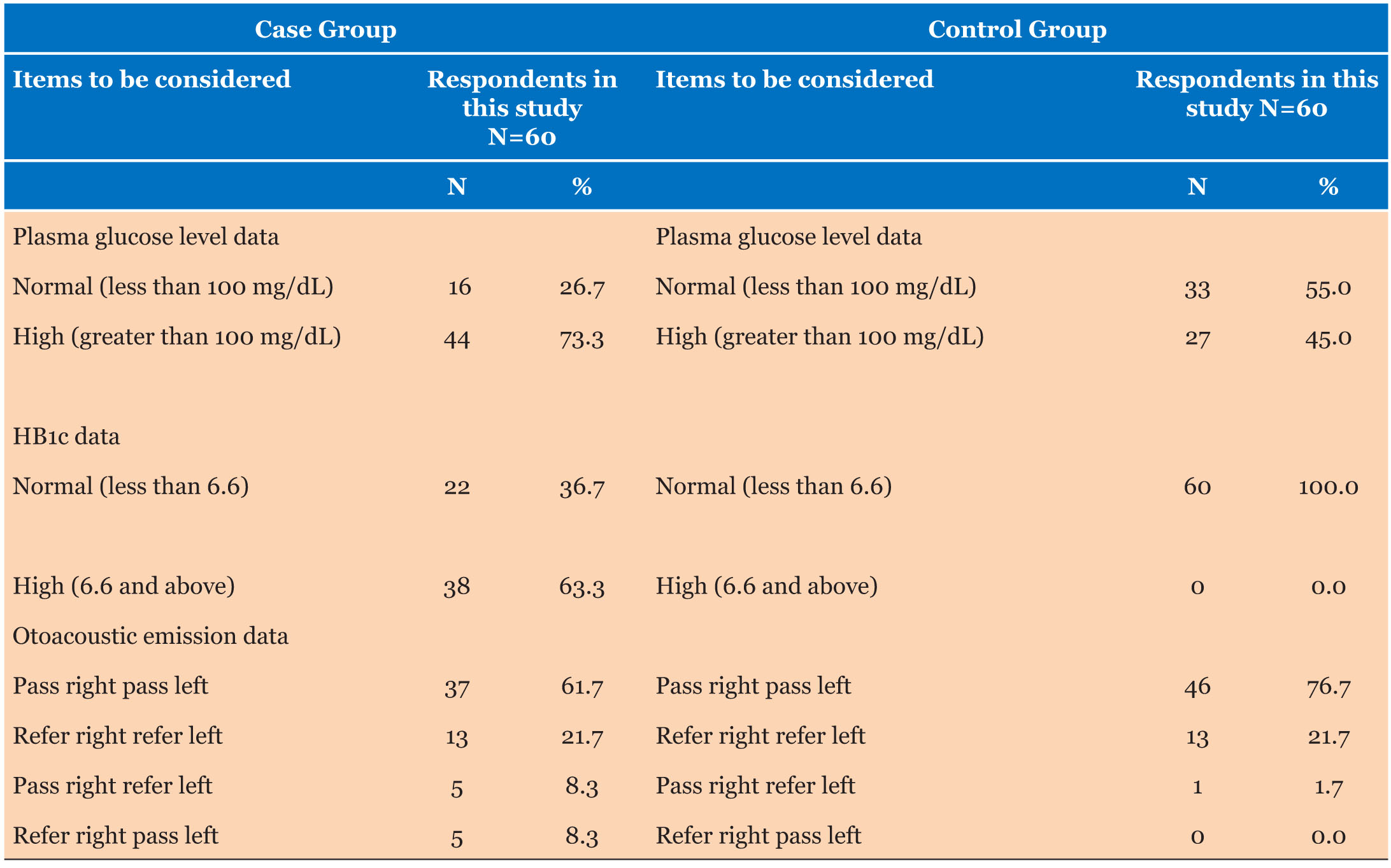
Plasma glucose level among the case group revealed that the majority of 44 (73.3%) of the participants had a high level of plasma glucose while on 16 (26.6%) had a normal level of plasma glucose. In terms of the control group, the data revealed that 55% (33) of the participants had a normal plasma glucose level, while 45% (27) of the participants had a high plasma glucose level.
HB1c assessment revealed that for the case group, 22 (36.7%) participants have normal while 38 (63.3%) participants have high HB1c. But for the control group, the data revealed that all participants, 60 (100%), have normal HB1c (Table 1 and Table 2).
Otoacoustic emission revealed that the case group has a pass in the right ear and pass in the left ear of 61.7% respectively, refer in the right ear and refer in the left ear of 21.7% respectively, pass in the right ear and refer in the left ear of 8.3% respectively, refer in the right ear and pass in the left ear of 8.3% respectively. The following results were revealed for the control group; pass in right ear and pass in left ear of 76.7%, refer in right ear refer in left ear of 21.7%, and pass in right ear refer in left ear of 1.7%.
ASSR; ASSR-audiometry; and ASSR-audiometry Rt an Lt for both groups
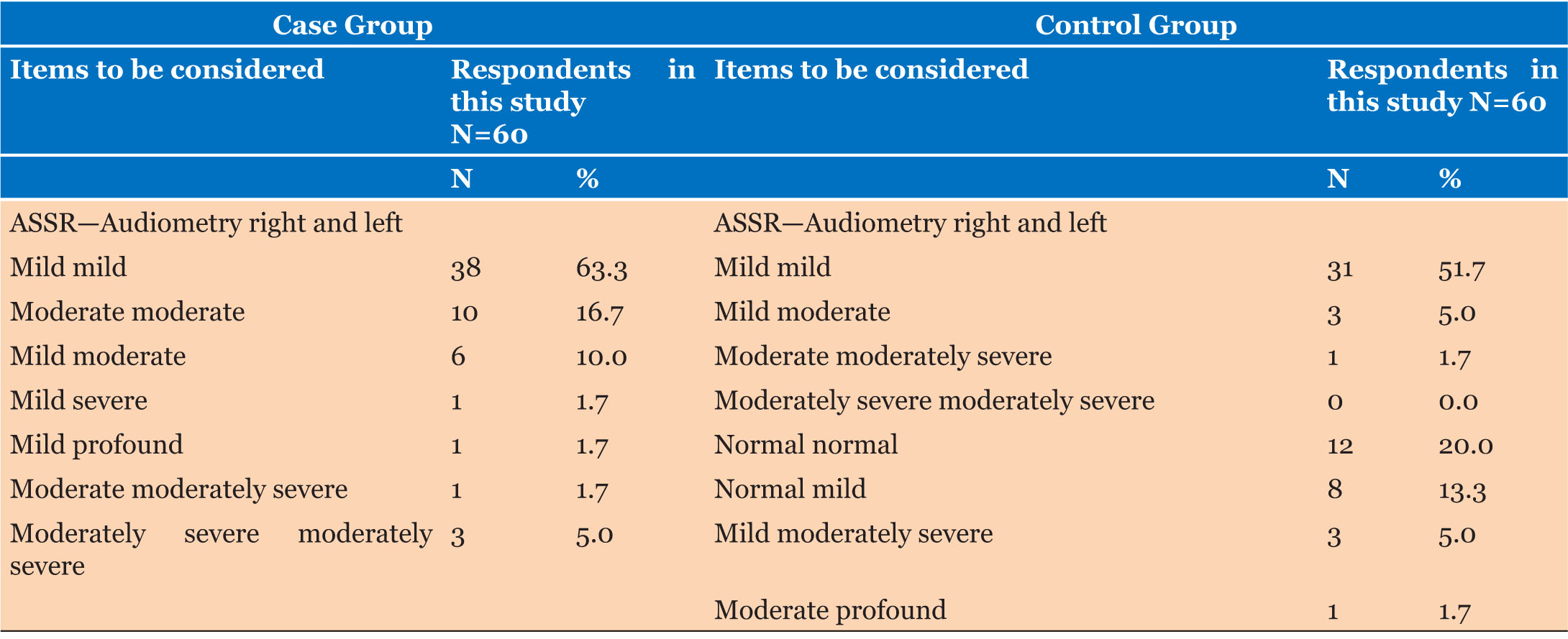
Auditory steady-state response audiometry for the two ear (right and left) for both the Case and Control groups revealed that those in the case group with mild mild audiometry are higher at 63.3%, those with moderate moderate were 16.7%, those with mild moderate were 10%, while those with moderately severe moderately severe were 5%. Furthermore, data from the control group revealed that those with mild audiometry for both ears were also higher at 51.7%, those with mild moderate were 5%, those with normal normal were 20%, those with normal mild were 13.3%, while those with mild moderately severe were 5% (Table 3 and Table 4).
DISCUSSION
Diabetes mellitus is known to affect the nervous system; sensory, motor, and autonomic divisions, including the auditory pathway [31]. Jardao was the first to report hearing loss in a diabetic patient [32].
The pattern of hearing loss in diabetics is commonly bilateral, progressive sensorineural hearing loss to all frequencies of sound, but particularly high frequency sounds [31],[33].
Diabetes mellitus has been isolated as an independent etiology of hearing loss [34].
Hearing loss in diabetics as mentioned above could be bilateral and progressive sensorineural could be erroneously diagnosed as presbycusis in elderly patients with diabetes. However, hearing loss is more severe than is usually observed for that age [8]. Some studies also listed diabetes as a known cause of sudden unilateral hearing loss [12].
The incidence of diabetes continues to increase exponentially across the globe [31],[35],[37]and a plethora of individuals are progressing toward undiagnosed diabetes and unawareness of complications [31].
Many theories have been postulated as the etiology of hearing loss in diabetes, the pathophysiological pathways include microangiopathy, deposition if advanced glycosylated end products and oxidative stress all culminating in metabolic dysfunction ultimately affecting auditory function and hearing loss [11],[14],[36],[37].
Due to the burden of diabetes and its strong association with hearing loss and its negative synergistic impact on quality of life, it is imperative to increase screening programs to curb the growing tide of undiagnosed and untreated diabetes and hearing loss as it’s complication [37].
In the screening for hearing loss, 3 assessment tools are usually used. Pure tone audiometry used to determine a hearing threshold, i.e., the softest sound audible to the person being studied. Otoacoustic emissions (OAE) used to evaluate the status of the cochlea, particularly the outer hair cell, and lastly, auditory brain stem response audiometry is a neurological test used to determine the retro-cochlear part of the auditory pathway, up to the brain stem [12]. However, in our study auditory steady-state response audiometry (ASSR) was used in place of pure tone audiometry as many studies have classified ASSR to be on par or superior to PTA [38],[39],[40].
The majority of participants in the test group and control group are in the age range of 40–49 years (Table 5), accounting for 33.5% and 25%, respectively. Similar findings were observed in a study with a demographic close to ours [10],[41].
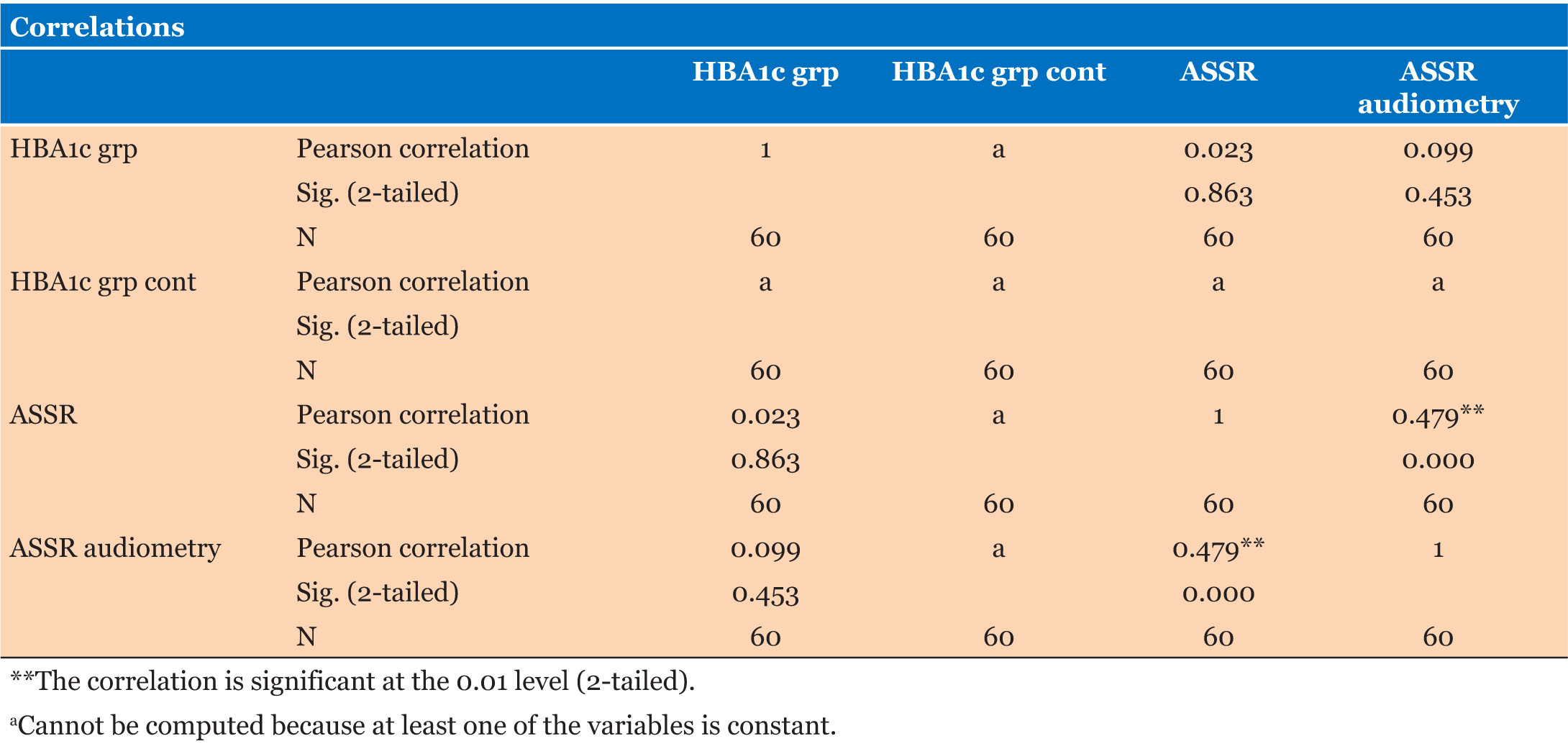
Our study has an equal number of sexes in the case group and more females in the control group (Figure 1). There is conflicting information on the correlation between sex and hearing loss in diabetics. A report does not show a relationship between hearing loss and sex [33], some reported hearing loss to be more common in diabetic women compared to their male counterparts [41], while some reported hearing loss to be more common in male diabetics [8]. However, our study didn’t find any correlation between both variables.
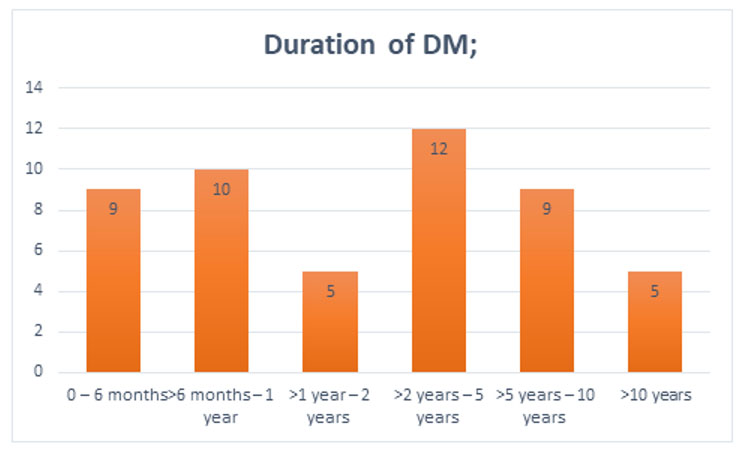
Threshold frequencies are known to be significantly higher in individuals who have had DM for more than five years [34], another study showed no correlation between them [10].
The fasting plasma glucose level was deranged in 73.3% and 45% of the case and control groups, respectively. Sixty-three percent (63%) of the case group had abnormal glycated hemoglobin (Table 1). Studies show that there is no correlation between fasting plasma glucose and hearing loss [10]; however, persistently high or deranged plasma glucose (glycated hemoglobin) is associated with sensorineural hearing loss in diabetic patients [42]. Our study showed no correlation between hearing loss and glycated hemoglobin levels.
The otoacoustic emissions performed showed that at least 38.3% and 23.4% of the participants had a referral in at least one ear for the case and control groups, respectively, revealing that hearing loss is more common in people with diabetes. However, the analysis did not show a significant correlation between hearing loss and diabetes.
CONCLUSION
In conclusion, hearing loss is an established complication of type 2 diabetes, proactive measures should be taken to make early diagnosis, start treatment, and regular screening to prevent hearing loss in patients with type 2 diabetes.
REFERENCES
1.
Tabish SA. A. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci (Qassim) 2007;1(2):V–VIII.
[Pubmed]

2.
Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. [CrossRef]
[Pubmed]

3.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62–9. [CrossRef]
[Pubmed]

4.
Chen HY, Baumgardner DJ, Rice JP. Health-related quality of life among adults with multiple chronic conditions in the United States, Behavioral Risk Factor Surveillance System, 2007. Prev Chronic Dis 2011;8(1):A09.
[Pubmed]

5.
Pera PI. Living with diabetes: Quality of care and quality of life. Patient Prefer Adherence 2011;5:65–72. [CrossRef]
[Pubmed]

6.
Egede LE, Ellis C. Diabetes and depression: Global perspectives. Diabetes Res Clin Pract 2010;87(3):302–12. [CrossRef]
[Pubmed]

7.
8.
Taylor IG, Irwin J. Some audiological aspects of diabetes mellitus. J Laryngol Otol 1978;92(2):99–113. [CrossRef]
[Pubmed]

9.
Axelsson A, Fagerberg SE. Auditory function in diabetics. Acta Otolaryngol 1968;66(1):49–64. [CrossRef]
[Pubmed]

10.
Meena R, Sonkhya D, Sonkhya N. Evaluation of hearing loss in patients with type 2 diabetes mellitus. Int J Res Med Sci 2016;4(6):2281–7. [CrossRef]

11.
Ren H, Wang Z, Mao Z, et al. Hearing loss in type 2 diabetes in association with diabetic neuropathy. Arch Med Res 2017;48(7):631–7. [CrossRef]
[Pubmed]

12.
Fukui M, Kitagawa Y, Nakamura N, et al. Idiopathic sudden hearing loss in patients with type 2 diabetes. Diabetes Res Clin Pract 2004;63(3):205–11. [CrossRef]
[Pubmed]

13.
Cheng YJ, Gregg EW, Saaddine JB, Imperatore G, Zhang X, Albright AL. Three decade change in the prevalence of hearing impairment and its association with diabetes in the United States. Prev Med 2009;49(5):360–4. [CrossRef]
[Pubmed]

14.
Uchida Y, Sugiura S, Ando F, Nakashima T, Shimokata H. Diabetes reduces auditory sensitivity in middle-aged listeners more than in elderly listeners: A population-based study of age-related hearing loss. Med Sci Monit 2010;16(7):PH63–8.
[Pubmed]

15.
Lerman-Garber I, Cuevas-Ramos D, Valdés S, et al. Sensorineural hearing loss—A common finding in early-onset type 2 diabetes mellitus. Endocr Pract 2012;18(4):549–57. [CrossRef]
[Pubmed]

16.
Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: Audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008;149(1):1–10. [CrossRef]
[Pubmed]

17.
Idugboe OJ, Kolawole BA, Totyen EL. Hearing threshold level among adult diabetics in South-Western Nigeria. J Otolaryngol Rhinol 2018;4(2):051. [CrossRef]

18.
Uju IM, Simeon A. Type 2 diabetes mellitus and hearing impairment as seen in a tertiary hospital in Port Harcourt. Int J Otorhinolaryngol Head Neck Surg 2021;7(4):585–91. [CrossRef]

19.
Harner SG. Hearing in adult-onset diabetes mellitus. Otolaryngol Head Neck Surg 1981;89(2):322–7. [CrossRef]
[Pubmed]

20.
Dalton DS, Cruickshanks KJ, Klein R, Klein BE, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care 1998;21(9):1540–4. [CrossRef]
[Pubmed]

21.
Hodgson MJ, Talbott E, Helmkamp JC, Kuller LH. Diabetes, noise exposure, and hearing loss. J Occup Med 1987;29(7):576–9.
[Pubmed]

22.
Thimmasettaiah NB, Shankar R, Ravi GC, Reddy S. A one year prospective study of hearing loss in diabetes in general population. Trans Biomed 2012;3(2):1–7. [CrossRef]

23.
Celik O, Yalçin S, Celebi H, Oztürk A. Hearing loss in insulin-dependent diabetes mellitus. Auris Nasus Larynx 1996;23:127–32.
[Pubmed]

24.
Nageris B, Hadar T, Feinmesser M, Elidan J. Cochlear histopathologic analysis in diabetic rats. Am J Otol 1998;19(1):63–5.
[Pubmed]

25.
Makishima K, Tanaka K. Pathological changes of the inner ear and central auditory pathway in diabetics. Ann Otol Rhinol Laryngol 1971;80(2):218–28. [CrossRef]
[Pubmed]

26.
Kurt E, Öztürk F, Günen A, et al. Relationship of retinopathy and hearing loss in type 2 diabetes mellitus. Ann Ophthalmol 2002;34(3):216–22. [CrossRef]

27.
Loader B, Stokic D, Riedl M, et al. Combined analysis of audiologic performance and the plasma biomarker stromal cell-derived factor 1a in type 2 diabetic patients. Otol Neurotol 2008;29(6):739–44. [CrossRef]
[Pubmed]

28.
Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol 2003;24(3):382–6. [CrossRef]
[Pubmed]

29.
Jorgensen MB, Buch NH. Studies on inner-ear function and cranial nerves in diabetics. Acta Otolaryngol 1961;53:350–64. [CrossRef]
[Pubmed]

30.
32.
Ali A, Nisar J. Clinical evaluation and incidence of sensorineural. International Journal of Current Advanced Research 2020;9(06(D)):22552–4. [CrossRef]

33.
Maia CAS, de Campos CAH. Diabetes mellitus as etiological factor of hearing loss. Braz J Otorhinolaryngol 2005;71(2):208–14. [CrossRef]
[Pubmed]

34.
35.
Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Characterization of hearing loss in aged type II diabetics. Hear Res 2006;211(1–2):103–13. [CrossRef]
[Pubmed]

36.
Hong O, Buss J, Thomas E. Type 2 diabetes and hearing loss. Dis Mon 2013;59(4):139–46. [CrossRef]
[Pubmed]

37.
Komazec Z, Lemajić-Komazec S, Jović R, Nadj C, Jovancević L, Savović S. Comparison between auditory steady-state responses and pure-tone audiometry. Vojnosanit Pregl 2010;67(9):761–5. [CrossRef]
[Pubmed]

38.
Ahn JH, Lee HS, Kim YJ, Yoon TH, Chung JW. Comparing pure-tone audiometry and auditory steady state response for the measurement of hearing loss. Otolaryngol Head Neck Surg 2007;136(6):966–71. [CrossRef]
[Pubmed]

39.
Yang XP, Fan LH, Zhou XR, Zhu GY. Comparison of thresholds acquired with ASSR and PTA in normal-hearing subjects. [Article in Chinese]. Fa Yi Xue Za Zhi 2008;24(4):248–51.
[Pubmed]

40.
Misra V, Agarwal CG, Bhatia N, Shukla GK. Sensorineural deafness in patients of type 2 diabetes mellitus in Uttar Pradesh: A pilot study. Indian J Otolaryngol Head Neck Surg 2013;65(Suppl 3):532–6. [CrossRef]
[Pubmed]

41.
Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol 1993;107(3):179–82. [CrossRef]
[Pubmed]

42.
Kim MB, Zhang Y, Chang Y, et al. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int J Epidemiol 2017;46(2):717–26. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Moses Ayodele Akinola - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Akolade Idowu - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Akindele Emmanuel Ladele - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Babatunde Akinola Bamigboye - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Oluwapelumi Olusoga-Peters - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Oluseyitan Adesegun - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Oladapo Abayomi Somefun - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Moses Ayodele Akinola et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.