Case Report
Ancient cervical vagal schwannoma and an interposition great auricular to vagus nerve graft
1 Department of Clinical Surgical Sciences, University of West Indies, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
2 Department of Clinical Surgical Sciences, University of West Indies, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
3 Department of Clinical Surgical Sciences, University of West Indies, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
4 Department of Clinical Surgical Sciences, University of West Indies, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
5 Department of Pathology, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
6 Department of Clinical Surgical Sciences, University of West Indies, Eric Williams Medical Sciences Complex, Champs Fleur, Trinidad and Tobago
Address correspondence to:
Nicholas Figaro
Department of Surgery, Eric Williams Medical Sciences Complex, Uriah Butler Highway, Champ Fleurs,
Trinidad and Tobago
Access full text article on other devices

Access PDF of article on other devices

Article ID: 100011O04NF2021
doi: 10.5348/100011O04NF2021CR
How to cite this article
Figaro N, Ramoutar R, Richards D, Arozarena R, Geelal M, Juman S. Ancient cervical vagal schwannoma and an interposition great auricular to vagus nerve graft. Edorium J Otolaryngol 2021;5:100011O04NF2021.ABSTRACT
Introduction: Schwannomas of the cervical vagus nerve are exceedingly rare and have a myriad of clinical presentations depending on the tumor size and its proximity to neurovascular structures. We report a rare case of a cervical vagal schwannoma that was successfully treated with en bloc resection and reconstruction with a great auricular to vagus nerve graft.
Case Report: A 42-year-old male presented with an asymptomatic, right lateral neck mass. Magnetic resonance imaging revealed a lesion 2.4 cm × 1.4 cm × 4.0 cm in size, located between the carotid arteries and internal jugular vein displacing the internal carotid artery anteromedially. En bloc resection of the lesion and reconstruction of the vagus nerve with a great auricular interposition graft was performed.
Conclusion: Vagal schwannomas are rare, benign neurogenic tumors. Complete surgical excision is the mainstay of treatment. When a safe surgical plane cannot be identified between the neurogenic tumor and the main nerve trunk, en bloc resection with reconstruction should be considered.
INTRODUCTION
Schwannomas also known as neurilemmomas are benign neoplasms derived from myelinated Schwann cells [1]. These nerve sheath tumors can occur throughout the body, with approximately one-third found in the head and neck region in individuals between the ages of 30 and 60 [2]. Schwannomas of the cervical vagus nerve are exceedingly rare and have a myriad of clinical presentations depending on the tumor size and its proximity to neurovascular structures [3]. These clinical features can range from a small, asymptomatic lateral neck swelling to a large cervical lesion causing dysphonia, dysphagia, upper airway obstruction and pain [4],[5]. The treatment of choice for vagal schwannomas is complete surgical excision with preservation of the neural integrity when possible [5]. We report a case of a 42-year-old male patient with a cervical vagal schwannoma who was successfully treated with en bloc removal of the lesion along with vagal nerve sacrifice and an interposition great auricular nerve graft.
CASE REPORT
A 42-year-old male was referred to our outpatient clinic with a gradually progressive asymptomatic swelling on the right side of the neck for 2 years. The patient did not experience any voice changes, difficulty swallowing nor had any comorbidities. Physical examination revealed a firm, non-tender lesion in the right level 2 cervical region measuring approximately 3 × 2 cm. The mass was mobile in the transverse direction only and upon palpation a paroxysmal cough was elicited. There was no visible swelling in the oral cavity or oropharynx and flexible laryngoscopy showed bilateral vocal cord mobility.
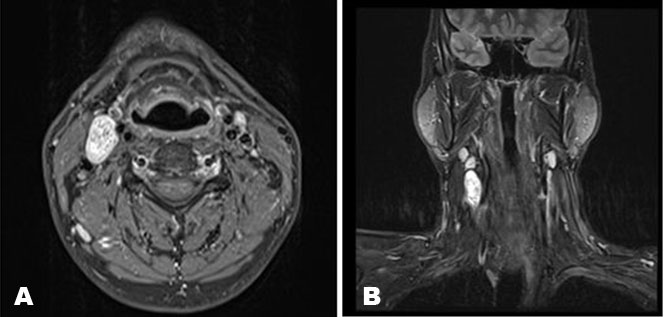
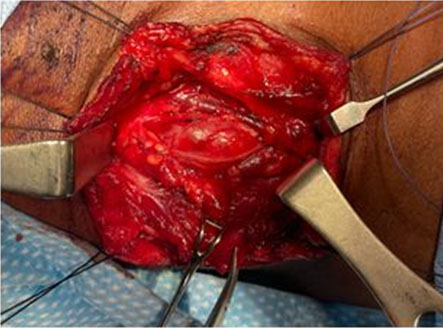
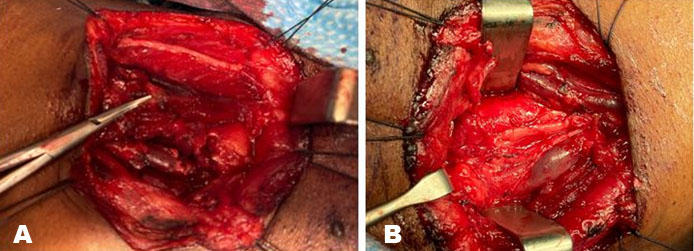
Ultrasound of the neck showed a well-circumscribed, solid mass approximately 3 cm in diameter on the upper right side of the neck subjacent to the carotid bifurcation. There was no significant internal vascularity within the lesion. Contrast enhanced magnetic resonance imaging (MRI) of the neck showed a well-defined, heterogeneously hyperintense lesion in the right anterior neck at the level of C4/C5 (Figure 1A and Figure 1B). The mass was 2.4 cm × 1.4 cm × 4.0 cm in size located between the carotid arteries and internal jugular vein, displacing the internal carotid artery anteromedially. The patient was diagnosed with a vagal schwannoma. Following extensive discussion with the patient and relatives, the decision was made to perform surgical excision. Under general anesthesia, a transverse cervical incision was made at the upper border of the thyroid cartilage. The carotid sheath was opened (Figure 2) and proximal and distal control of the vasculature was obtained. A tan colored, ovoid-shaped mass was identified in continuity with the vagus nerve (Figure 3).
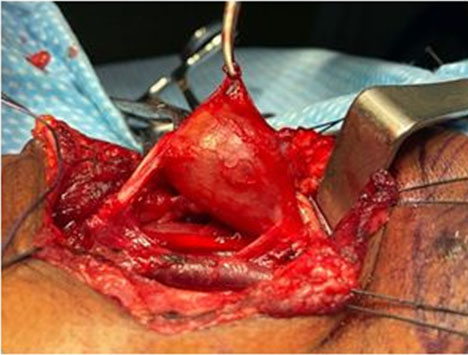
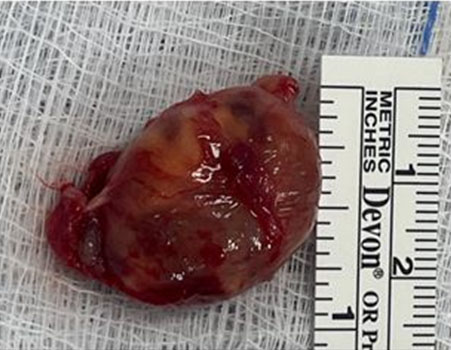
Using intraoperative nerve monitoring and surgical loupes an attempt was made to identify a surgical plane between the splayed nerve trunk and the tumor. Despite all efforts the vagus nerve could not be separated from the tumor, thus a gross total resection of the tumor was performed leaving a 1 cm sleeve of vagus nerve on either end (Figure 4) and a great auricular interposition nerve graft was fashioned using microsurgical techniques (Figure 5A and Figure 5B).
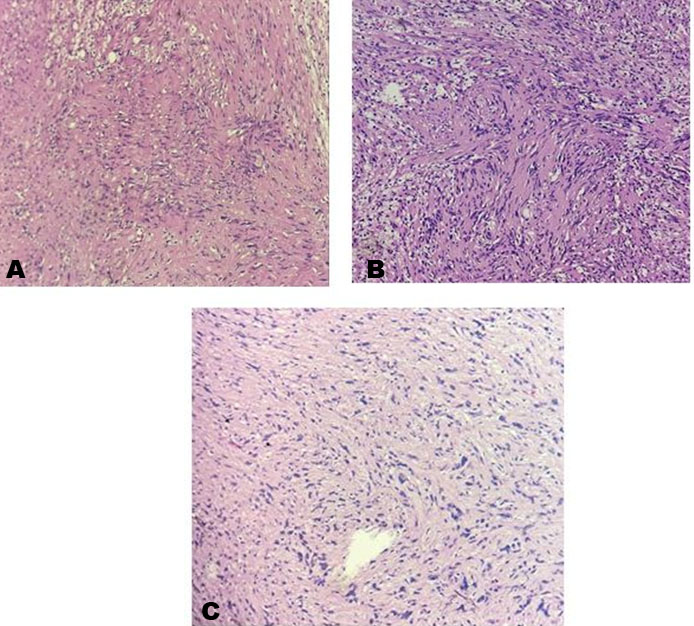
Despite the notable hoarseness, the patient had an uneventful hospital stay and was discharged on post-operative day three. Pathological examination confirmed the diagnosis of a benign vagal schwannoma. Microscopically, the tumor was composed of spindle cells with elongated wavy nuclei, interspersed with collagen fibers. Verocay bodies were identified and the tumor cells displayed degenerative nuclear atypia, consistent with an ancient schwannoma (Figure 6A, Figure 6B, Figure 6C). At outpatient follow-up, six months after surgery, the patient had no significant complaints. Despite having right vocal cord paralysis, his voice improved as the left vocal cord has compensated for the phonatory gap.
DISCUSSION
Schwannomas are indolent growing, well defined, solitary neoplasms arising from the nerve sheath of any cranial nerve (with the exception of the optic and olfactory nerve) or spinal nerve [6]. First identified as a pathological entity by Uruguayan neuropathologist, Jose Verocay, these rare lesions account for 1% of all head and neck tumors [3]. The preoperative diagnosis of a vagal schwannoma is challenging. The clinical presentation of an asymptomatic lateral neck mass is common among many other cervical pathologies. Inflammatory lymphadenopathy, branchial cleft cyst, malignant lymphoma, carotid body tumor, and metastatic cervical adenopathy are some of the more common neck lesions that present similarly to the vagal schwannoma and should be in the differential diagnosis of any head and neck surgeon [7]. Occasionally, the patient may be symptomatic, in this situation the most common presenting complaint is hoarseness followed by a paroxysmal cough on palpation of the neck lump [2]. The paroxysmal cough is considered a pathognomonic sign for a vagal schwannoma, this feature was elicited in our index case [2].
Radiological imaging is crucial in the diagnosis of this neurogenic tumor. Knowledge of the relationship of the cervical mass to the surrounding neurovascular and muscular structures are critical from both a diagnostic and management perspective [4]. Typically, a vagal schwannoma appears as a well-defined ovoid enhancing lesion in-between the internal jugular vein (IJV) and carotid artery [7]. Furukawa et al. proposed that the pattern of vascular displacement within the carotid space can allude the clinician to the nerve of origin of the schwannoma [8]. As per the index case, a vagal schwannoma generally displaces the IJV laterally and the carotid arteries anteromedially [8]. On the other hand, a sympathetic chain schwannoma does not separate the IJV and carotid artery but rather displaces both anteriorly [8].
Once imaging identifies a vagal schwannoma, treatment is dictated by symptomology, size, location, the patient’s age, and general medical condition [5]. Surgical treatment has the potential for significant morbidity like hoarseness, impaired cough mechanism, and chronic aspiration, therefore it is not uncommon for asymptomatic patients with smaller lesions to pursue an initial period of conservative management [3]. However, several authors have estimated the rate of tumor growth to be as much as 3 mm per year, and as such, most patients will require surgical treatment after the period of surveillance [9].
The treatment of choice for a vagal schwannoma is complete surgical excision with preservation of the neural continuity [10]. However, the intimacy with which the vagal nerve fibers are related to the tumor capsule makes excision of the schwannoma extremely challenging. Intracapsular enucleation of the vagal tumor described by Fujino et al. has become the standard surgical technique utilized [11]. This approach entails meticulous intracapsular dissection in an attempt to shell out the tumor from the vagus nerve. This method generally leaves the tumor capsule behind along with the uncut nerve fibers. This technique has been documented to preserve nerve function in up to a third of the surgical cases and have miniscule tumor recurrence rate [12]. Unfortunately, in our case, despite attempting the intracapsular enucleation technique, it was impossible to identify a safe surgical plane to separate the vagus nerve from the tumor, as a result an en bloc resection of the tumor with nerve sacrifice was performed. The interposition nerve graft facilitates electromyographic recovery of the vocalis muscle which can potentially maintain its muscle bulk and tone and decrease post-operative morbidity [13].
Schwannomas and neurofibromas are the most common benign tumors of the peripheral nerve sheath [14]. Generally, discerning these clinically similar tumors, when classic morphological features are present, can be done confidently with Hematoxylin and Eosin staining, as per the index case [14],[15]. However, in some instances these peripheral nerve sheath tumors may exhibit morphological variability and overlap, making histological diagnosis challenging. In such cases, the use of immunohistochemical markers like S-100, Calretinin, EMA, and Leu-7 can aid the pathologist’s diagnosis [14],[15].
Classically, schwannomas have two distinct architectural areas, Antoni A and Antoni B [16]. Generally, Antoni A areas are compact, exhibit high cellularity and Verocay bodies, whereas Antoni B areas are loosely arranged with less cellularity [17]. There are several subtypes of schwannomas, namely, conventional, cellular, plexiform, melanotic, epithelioid, and ancient [5]. Our case presents an ancient schwannoma, a term that was coined by Ackerman and Taylor to describe the degenerative changes or “aging” of the schwannoma [16]. These degenerative changes include the formation of cysts, edema, fibrosis, and wide areas of hyalinized matrix [17]. Although there is a paucity of data documenting ancient vagal schwannomas, they are believed to exhibit similar clinical behavior to that of the conventional subtype [5].
CONCLUSION
Despite the vast differential for a lateral neck mass, a vagal schwannoma should be considered particularly when associated with hoarseness and a paroxysmal cough on palpation. Radiological imaging is paramount for preoperative diagnosis and can aid in discerning the nerve of origin within the carotid space. Complete surgical excision is the mainstay of treatment. Meticulous intracapsular enucleation with preservation of vagal nerve continuity can significantly decrease postoperative morbidity. When a safe surgical plane cannot be identified between the neurogenic tumor and the main nerve trunk, en bloc resection with reconstruction should be considered.
REFERENCES
1.
Ansari I, Ansari A, Graison AA, Patil AJ, Joshi H. Head and neck schwannomas: A surgical challenge—A series of 5 cases. Case Rep Otolaryngol 2018;2018:4074905. [CrossRef]
[Pubmed]

2.
Singh ID, Galagali JR. Cervical vagal schwannoma presenting as paroxysmal cough—A case report. J Otolaryngol ENT Res 2017;7(3):00206. [CrossRef]

3.
Mat Lazim N. Challenges in managing a vagal schwannomas: Lesson learnt. Int J Surg Case Rep 2018;53:5–8. [CrossRef]
[Pubmed]

4.
Singh I, Sharma SA, Manu V, Phogat D. Cervical vagal schwannoma mimicking tonsillar mass. J Otolaryngol ENT Res 2018;10(3):142–4. [CrossRef]

5.
Sandler ML, Sims JR, Sinclair C, et al. Vagal schwannomas of the head and neck: A comprehensive review and a novel approach to preserving vocal cord innervation and function. Head Neck 2019;41(7):2450–66. [CrossRef]
[Pubmed]

6.
Verma RK, Sunku SK, Bal A, Panda NK. Cervical vagal schwannoma and cable grafting of vagus nerve: A rare case report and review of literature. Otolaryngol Online J 2014;4(4):1–13.

7.
Cavallaro G, Pattaro G, Iorio O, Avallone M, Silecchia G. A literature review on surgery for cervical vagal schwannomas. World J Surg Oncol 2015;13:130. [CrossRef]
[Pubmed]

8.
Chiofalo MG, Longo F, Marone U, Franco R, Petrillo A, Pezzullo L. Cervical vagal schwannoma. A case report. Acta Otorhinolaryngol Ital 2009;29(1):33–5.
[Pubmed]

9.
Furukawa M, Furukawa MK, Katoh K, Tsukuda M. Differentiation between schwannoma of the vagus nerve and schwannoma of the cervical sympathetic chain by imaging diagnosis. Laryngoscope 1996;106(12 Pt 1):1548–52. [CrossRef]
[Pubmed]

10.
Basavaraj, Rajendrakumar NL, Henmath PN, Umamaheshwari KB, Nishanth RK. Ancient schwannoma of the cervical vagus nerve: A rare benign neurogenic tumor. Int J Med Res Health Sci 2015;4(1):219–2.

11.
Fujino K, Shinohara K, Aoki M, Hashimoto K, Omori K. Intracapsular enucleation of vagus nerve-originated tumors for preservation of neural function. Otolaryngol Head Neck Surg 2000;123(3):334–6. [CrossRef]
[Pubmed]

12.
Valentino J, Boggess MA, Ellis JL, Hester TO, Jones RO. Expected neurologic outcomes for surgical treatment of cervical neurilemomas. Laryngoscope 1998;108(7):1009–13.
[Pubmed]

13.
Pyle GM, Ford CN. Treatment of vagal injuries in resection of large glomus tumors. Oper Tech Otolaryngol - Head Neck Surg 1994;5(2):54–62.

14.
Guedes-Corrêa JF, Cardoso RSV. Immunohistochemical markers for schwannomas, neurofibromas and malignant peripheral nerve sheath tumors—What can the recent literature tell us? Arq Bras Neurocir Brazilian Neurosurg 2018;37(2):105–12. [CrossRef]

15.
Park JY, Park H, Park NJ, Park JS, Sung HJ, Lee SS. Use of calretinin, CD56, and CD34 for differential diagnosis of schwannoma and neurofibroma. Korean J Pathol 2011;45(1):30–5.

16.
Krishnamurthy A, Ramshankar V, Majhi U. Ancient cervical vagal schwannoma: A diagnostic challenge. Indian J Surg Oncol 2013;4(3):284–6. [CrossRef]
[Pubmed]

17.
Sreevatsa MR, Srinivasarao RV. Three cases of vagal nerve schwannoma and review of literature. Indian J Otolaryngol Head Neck Surg 2011;63(4):310–2. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Nicholas Figaro - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Rickhi Ramoutar - Acquisition of data, Drafting the article, Final approval of the version to be published
David Richards - Analysis of data, Interpretation of data, Drafting the article, Final approval of the version to be published
Rodolfo Arozarena - Analysis of data, Revising it critically for important intellectual content, Final approval of the version to be published
Mala Geelal - Analysis of data, Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Solaiman Juman - Analysis of data, Revising it critically for important intellectual content, Final approval of the version to be published
Data Availability StatementThe corresponding author is the guarantor of submission.
Consent For PublicationWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Competing InterestsAuthors declare no conflict of interest.
Copyright© 2021 Nicholas Figaro et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.